FDA warns consumers to stop using and purchasing around 26 over-the-counter eyedrops products due to a potential risk of eye infection which may lead to partial vision loss or blindness. The agency investigators have found unsanitary conditions in the manufacturing facility and bacterial tests from the environmental sampling of critical drug production areas in the facility came back positive which led to FDA advising manufacturers to recall these products last October 25th.
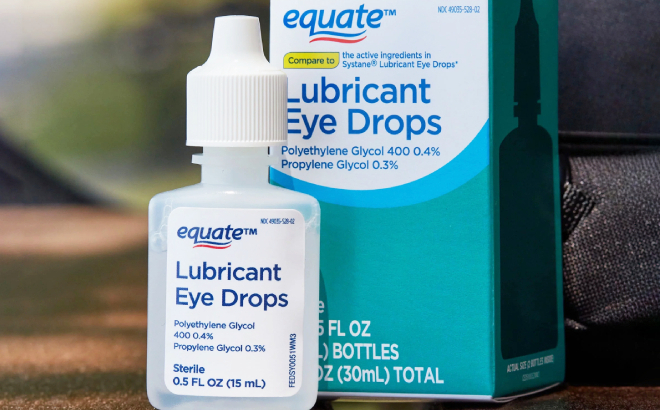
The eyedrops and gels included are marketed under the CVS Health, Leader (Cardinal Health), Rugby (Cardinal Health), Rite Aid, Target Up & Up, and Velocity Pharma. Some of the symptoms of eye infection include eye pain or discomfort, discharge, blurry vision, and redness or swelling of the eye or eyelid. If you have felt any adverse effects while using the products, you can report to FDA’s MedWatch Adverse Event Reporting program.
All consumers who have purchased the contaminated eye products are encouraged by FDA to properly discard them by taking them to a drug take-back site or check if the product is included on the F.D.A.’s “flush list” of drugs which can be safely discarded at home. FDA reports that CVS, Rite Aid, and Target have removed the products from their shelves and websites while products branded as Leader, Rugby and Velocity may still be available in-stores and online and should not be purchased. To see the full report and complete list of the eyedrop retailers and products, click here!

































Helpful info! Thanks 🙏🏽
Most welcome Chandira!
wow, thanks for the message!!
Most welcome!
Thank you for letting us know. This is good to know.
Glad you find this helpful Liliana!
Nice post! This is my fav❤️ so many times ppl don’t know.. also recently 300 otc meds were recalled from family dollar, dollar general & other stores. A lot of those meds are cold meds (for winter) & kid’s products! Plz post that for the community. Thanks for u do!❤️
Thank YOU so much for sharing that Miller! We’ll look into that as soon as we can.
Good to know!